TOWARDS THE ABSOLUTE ZERO .
TECHNICAL NOTES ON THE HISTORY OF COLD AND THE ACHIEVEMENT OF LOW TEMPERATURES .
( Dr. Leopoldo Avalle - Genci Tepelena ).
Describe, even briefly, the history of cryosurgery, involves dutifully talk of scientists, physical, chemists and engineers, who opened the present to use of tools that are included in the operating room as normal. The work of these pioneers has provided the tools that surgeons use for their contributions to cryosurgery. In this brief review will not be cited scientists while others are remembered for their research and discoveries. This choice is, of course, my responsibility, and for the reader who wishes to deepen the topic refer to publications most up to date and better detailed.
Let me therefore take this opportunity to thank Dr. Roberto Renzetti that in his articles the low-temperature physics ( Part I and Part II ) was able to describe the evolution of events with large capacity descriptive and scientific.
In this bibliography we have enclosed the necessary references which the interested reader can consult.
Our attention will focus on the figure of Professor Linde. He was, in our opinion, the engineer who has made industrial and commercial then the production of cryogenic gases. In particular, liquid nitrogen, which, as we know, is the fluid used in modern cryosurgery.
We begin, therefore, our historical investigation.
To make it easier to understand what a thermodynamic cycle cryogenic (to lower the temperature to a given object) it is useful to speak of the Carnot cycle for reasons strictly thermodynamic was considered to peak performance. It is therefore a theoretical cycle to which all other cycles are approaching, tend to it , but cannot overcome.
We leave to the reader the choice to reading it.
The refrigeration cycle. Carnot cycle.
It ' a thermodynamic cycle employed in order to subtract heat to a body that should bring and keep at a temperature lower than ambient. Very often these are spaces within which are located the bodies to cool down, or it comes to getting the bodies in a particular state as ice water, air and other gases in liquid, solid carbon dioxide ( or dry ice).
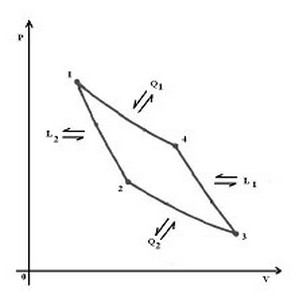 The heat removed in such bodies is usually moved to the external environment to them. The easiest way for the aforementioned transfer of heat is provided by the cycle of N. L. Sadi Carnot (1824), reversed (see figure below reproduced).
The heat removed in such bodies is usually moved to the external environment to them. The easiest way for the aforementioned transfer of heat is provided by the cycle of N. L. Sadi Carnot (1824), reversed (see figure below reproduced).
Along the cycle 1-4-3-2 you have the following: 1-4 isothermal expansion ; 4-3 , adiabatic expansion ; 3-2 , isothermal compression ; 2-1, adiabatic compression .
Energy exchanges with the outside world are, in order : supply of heat Q 1, L 1 obtaining work , heat dissipation Q 2 ; supply of labor L 2.
It ' well known that the useful work is given by the area of the cycle i.e. from :
L = L 1 - L 2 = Q 1 - Q 2 and that performance is:
h = L / Q 1 = ( Q 1 - Q 2 ) / Q 2 = 1 - Q 2/Q 1
The demand for labor L is emphasized by the effect refrigerator :
= Q2 / L = T2 / (T1 - T2)
ie at constant temperature T2 is greater the smaller the difference ( T1 - T2 ) and vice versa. From this stems the fundamental principle of cooling technique:
the cold coast much more expensive
than the lower the temperature
T2 , at which you want to get.
In practice, since heat exchanges require heat exchangers, the temperatures T 1 and T 2 will be respectively greater and less than the theoretical ones, therefore the refrigerating effect will be lower, i.e. the labor spent greater than the theoretical ones.
The additional power can also be supplied in a different way from the mechanical work. They differ in the technique therefore two different types of processes:
1 ) those that employ mechanical energy, in all compression machines;
2) those that use thermal energy in all absorption chillers and thermo-compression.
The Carnot cycle belongs to the first type. In it, however, there are two isothermal transformations, while in reality the body that transfers the heat cools and Q 2 that receives heat Q 1 heats up.
As is well known Carnot cycle is by its nature ideal: the different sequences studied and applied in practice tend to approach it.
European research on low temperatures (between mid-800 's and early 900's).
In those years, Van der Waals writes equation of gas (which bears his name). This equation whose function is to draw the theoretical representation of the gas to the physical reality was of immense utility for understanding the behavior of real gases. The size of the molecules and the intramolecular forces are the main factors corrective theoretical equation of the gases. These changes could explain the phenomena relating to the expansion.
Regnault in his studies on the expansion of gases due to the temperature, we are in the year 1847, had resulted in 1/273 the coefficient of expansion of air (per degree centigrade temperature supplied), which was determined to improve the first to Gay-Lussac estimated at 1/266.
Means that decreasing the temperature of a degree centigrade of a gas these decreases by 1/ 273 of the volume. It follows that in -273°C, the volume of each gas should be zero.
These, in a nutshell, are the reasons why the temperature of -273°C is called "absolute zero". And ' in fact an insurmountable barrier for obvious reasons volumetric.
Ultimately, according to the calculations of Regnault air was a little cool when coming out of a hole capillary expanded without doing work.
Thomson was thus that , using the experience experimental of Joule in a seven-year collaboration , led to the determination that when the molecules are separated due to the expansion , you need a mechanical work to win the small force of attraction that binds them and this involves an absorption of heat and then cooling . This is the Joule-Thomson effect which is essential for the liquefaction of air (see below machine of Linde ).
The machine which uses the Joule-Thomson effect is constructively very simple , although generally provides a very small effect . It is ultimately to replace the engine in the expansion valve Joule- Thomson.
Were made two patents are very similar in 1985 in Germany by Linde and Great Britain on behalf of Hampson .
At end of nineteenth century the advance in science begins to have support of big business. Change, of course, the pioneering approach that has characterized the beginning of the nineteenth century and together with theoretical studies on understanding the structure of matter and thermodynamics it will undertake the way toward absolute zero
LINDE
Professor Dr. Carl Paul Gottfried von Linde was born in Berndorf ( Franconia) on June 11, 1842 and died in Monaco of Bavaria November 16 , 1934 at the age of ninetytwo years. He studied and realized refrigeration techniques and technologies for gas separation .
Linde was a member of scientific and engineering associations , was also an eminent director of the German National Institute of Meteorology and the Bavarian Academy of Sciences and Humanities
 In 1861, to nineteen years old, Linde enrolled in a course of Engineering Swiss Federal Institute of Technology in Zurich. Among his teachers remember Rudolf Clausius, Gustav Zeuner and Franz Reuleaux.
In 1861, to nineteen years old, Linde enrolled in a course of Engineering Swiss Federal Institute of Technology in Zurich. Among his teachers remember Rudolf Clausius, Gustav Zeuner and Franz Reuleaux.
He graduated successfully at the beginning of 1864.
He started in the same year as an apprentice in a society spinning in Kottern in Kempten but remained only for a short time before moving. Initially towards Borsig in Berlin and then to the new works on locomotives of Krauss in Monaco of Bavaria, where he worked as head of the technical department.
In September of 1866 he married Helene Von, their marriage lasted fifty-three years and had six children.
In 1868 he heard about the opening of a new university in Monaco of Bavaria (Technische Hochschule) and immediately did apply for a job as a lecturer.
His request was immediately accepted and , given his young age (he was only twenty-six years), this was a clear signal of what will become the future of his career.
Linde in 1870 and 1871 published articles in the Bavarian Industry and trade journal describing his research findings with regard to refrigeration. The first refrigeration systems designed by Linde obtained a great commercial success.
In 1872 he became a professor of mechanical engineering. He was able to install an efficient laboratory in which, among his pupils, who worked with him we remember von Rudolf Diesel (the creator of the famous eponymous thermodynamic cycle and internal combustion engine ).
In the 1880s Linde is dedicating to manufacture and sell its machines for cold and, despite moments not really conducive to trade, in the early 1890s the new efficient refrigeration technology offers great benefits to breweries had sold 747 machines. Not only the breweries but new technology has found success in slaughterhouses and canning factories throughout Europe.
In 1890, Linde , has moved back to Monaco of Bavaria where he got his professorship. In 1894 he began work on a process for liquefying air. In 1901, Linde has started the construction of a car to get pure oxygen and nitrogen based on the fractional distillation of liquefied air. Since 1910, together with his son Carl Friedrich developed a process called Double - column Linde, whose variants are still in common use.
After a decade Linde withdrew from business to devote himself to research and in the first decade of the 1900s was able to liquefy air. Compressing it and then letting it expand quickly, making them suffer as a result of cooling. Scored in this way the oxygen and nitrogen from the liquid by subjecting it to a slow heating ( fractional distillation ). The use of oxygen found great used in oxyacetylene torch, invented in France in 1904, which revolutionized the cutting and welding of metals in the construction of ships, skyscrapers and other steel structures.
Linde, as we have seen, was not only a renowned engineer and a skilled engineer, he was also an outstanding entrepreneur. He has trained many successful companies in Germany and international, working effectively in order to maximize the potential of its patents. Linde has also founded the Linde Air Products Company in USA in 1907, a company in which the government of United States controls the in the 1940s what is now called Praxair.
In the years around 1910 began to transfer the responsibility of the company to his sons Friedrich and Richard and his son Rudolf Wucherer.
 Carl von Linde died (as mentioned above) in Monaco of Bavaria in November 1934 at the age of ninety two years.
Carl von Linde died (as mentioned above) in Monaco of Bavaria in November 1934 at the age of ninety two years.
His apparatus for liquefying air has used the cooling effect due to a compressed gas that expands (Effect of the Joule-Thomson) with a technique of heat exchange in countercurrent using the cold air produced to cool the ambient that enters into the machine. We will highlight the contribution that the machine Linde gave for the liquefaction of helium gas and the achievement of near temperature absolute zero .
Europe research center in the world on very low temperatures.
James Dewar (1842-1923), professor of chemistry at Cambridge, in 1878, gave a lecture at the Royal Institution in London. He used to present on Friday the experiments and scientific innovations. That year, he informed about the research of Cailletet Pictet, using a cryogenic machine they came from Paris as a gift of the patron Warren de la Rue.
Dewar did not give details of construction of the apparatus describing. This act had two important facts and as a result, precisely, it had to produce gas liquids in quantities moderately abundant and these gases must be kept for a reasonably long time to allow for the verification of a large audience.
In 1884 came the need to build a laboratory to create independence that university required.
Dewar realized that the instrument is still in use, and bears his name, the Dewar flask (also known as thermos or cryostat ). The objective of Dewar, however, was to liquefy the hydrogen (and therefore show ) but by some accounts on the latent heats of evaporation showed that the insulation systems of Cailletet would not have allowed to maintain liquid hydrogen. In 1892, he brought the changes that put him in a position to hold and keep the liquid hydrogen and showed his apparatus in its final form at a conference in January 1893. The novelty of the experience of these years, compared to what you had, is the use of vacuum insulation that much better before there was air in the cavity.
He used a machine that uses the Joule-Thomson effect.
Recall that the said equipment was already being used by Linde and that thanks to the German scientist contribution to the advancement of science cryogenic should not be forgotten.
Describe , using a synthetic scheme, the operation of said machine Linde ( or Hampson ).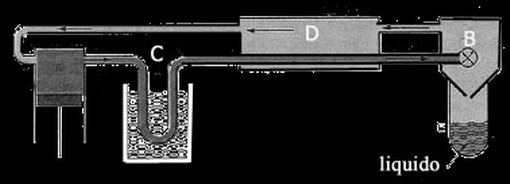
The evolving fluid cycling in the car going to the physical conditions later. Located in the container A is compressed and, after passing through a capacitor C, comes to a heat exchanger D from here to the container B in which is subject to expansion through a nozzle. In this process results in a slight lowering of the temperature so that the gas expands, in D, cools the incoming.
In this cycle, there is a continuous and progressive lowering of temperature in the fluid until the latter begins to liquefy and to concentrate in E.
Dewar had the feeling of being close to the goal. On July 9, the news came from Leiden which destroyed any hope: helium was liquefied by Professor Dr. Kamerling Onnes at the University of Leiden, July 9, 1908. Dewar was not a soul serene and gentle, he abandoned the research on cryogenic and became increasingly fractious and shady. He led a retired life. So, by losing a good teacher, Britain did not make more research on the low temperatures.
Kamerlingh Onnes in Leiden.
He was born in Graminga September 21, 1853 and died in Leiden on February 21, 1926.
It was a great experimental physicist who did not disdain, however, the theoretical approaches.
He was a kind-hearted and very patient which made the precision one of his important references, so that inaugurated his physics course with a lesson that was entitled precisely the importance of quantitative research in physics. He rigged his laboratory so that it was practical oriented research of the highest precision so that, apart from his personal achievements, those laboratories were for example the construction of several others in different parts of the world.
He worked closely with the industry. In his laboratory it blew glass and made precision instruments; was printed in a scientific journal, Communications of the Laboratory of Physics of the University of Leiden, updating in a comprehensible manner of every step that you walked on the road of low temperatures and which became an indispensable tool for every research laboratory that dealt of the same problems.
Helium extracted from some sands of monazite, he was in the meantime procured in large quantities by the interest of his brother who ran the Office of Commercial Information. As reported by Kamerlingh Onnes, the preparation of pure helium in large quantities became mainly a matter of perseverance and care. Meanwhile, the mechanical workshops had provided the design of the machine that was supposed to liquefy helium. It was a great system based on machine cyclic Linde that he was ready to operate in June 1908.
It was Onnes to focus its research on the properties of matter at those temperatures. One of the first research that was carried out from 1911 related to the anomaly of the resistance and resistivity of the conductors who seemed to decrease when the temperature dropped to zero at absolute zero ( this was a problem for Onnes that prevented him from working with thermometers use changes in the resistance of certain metals ), and with the commercial development of electricity, it was of interest general and industrial. The problem of resistance to low temperatures if the was placed also Dewar but had compounded than rather solve them.
The physical chemist Walther Nernst (1864-1941) formulated the hypothesis that the approaching absolute zero , the resistance was zero.
Kamerlingh Onnes began to search from there, the temperatures at which Dewar had left and, by its precise and rigorous method, published a series of memoirs on the experimental measurements carried out on the resistivity of various metals at very low temperatures. Here, too, several difficulties arose in the early experiences because it seemed that when the temperature decreased, the resistance is held constant. It was in a complete standstill without being able to support one or the other theory and/or measurement. There was, however, an observation decisive Onnes on samples of the same metal used. Since the same type of metal, the resistance (regardless vary by temperature ) varied from sample to sample and was lower as was the purest metal. This could only depend on the impurities contained in the sample and Onnes moved on this line of research. Therefore he chose the metals , with the technology of the time, you could better starting purify the gold that was better than platinum in this respect.
The experience with the gold going in the direction of what was claimed by Nernst, the resistivity decreased with decreasing temperature. With however a profound difference that Onnes not caught immediately because his ideas on the line of Nerst were an prejudice. Now say with Nerst that absolute zero the resistivity becomes nothing would remain if the fact was confirmed at the futility of it, given the difficulties to bring about some degree above the zero. To Look for what Onnes were finding however, that the resistivity becomes zero to temperatures useable with liquid helium, it was a phenomenon with profoundly different characteristics. In any case Onnes gave a formula for the behavior of the resistivity in the case of gold, which provided a resistivity that went to zero with absolute zero. And here it is useful to give a reference to theoretical physics that was claimed, although in a rather messy. To explain the significant decrease of resistance to virtually zero to 4.2°K and the fact that the resistance will remain at zero for lower temperatures, Onnes made use of the quantum theory of Planck.
Using the oscillators of the theory of specific heats of Einstein and Planck, and Lorentz's theory of electrons, Onnes presented his formula for the electrical conductivity as a function of temperature. At low temperatures were not electrons but the oscillators that tended to freeze, according to their frequencies derived from the specific heat.
Onnes stood for a whole year to work on mercury to understand that he the strange phenomena he had noticed and the existence of which gave a single signal in closing the memory of November 1911. Let us read Onnes in 1913 in three successive and long memories of February, March and May. Studying the properties of other materials at low temperatures also to discover the validity of Ohm's law. He was convinced that there is a transition point below which such a law is no longer valid and introduced (in memory of March) the term superconductivity K. Onnes was never able to solidify helium. The understanding of the problem came only after his death which occurred in 1926 when he had the Nobel Prize for Physics in 1913 (for his discovery of superconductivity in 1911 ).
Onnes received the Nobel Prize for physics at the age of 60 years.
 Kamerlingh Onnes , as well as his scientific efforts , he was a man devoted to his family and was always available in regard to those who needed it . He had a great personal charm and had philanthropic humanity . It 'been very active during and after the First World War to spreading the understanding of the differences political between scientists and rescuing children who are starving in countries that suffer from food shortages.
Kamerlingh Onnes , as well as his scientific efforts , he was a man devoted to his family and was always available in regard to those who needed it . He had a great personal charm and had philanthropic humanity . It 'been very active during and after the First World War to spreading the understanding of the differences political between scientists and rescuing children who are starving in countries that suffer from food shortages.
In 1887 he married Maria Adriana Wilhelmina Elisabeth Bijleveld , which was a great help to him in these activities and was able to create in his house a center well known for its hospitality and culture. They had a son, Albert, who became a high-ranking official in the capital city of The Hague.
Kamerlingh Onnes has always had poor health and , after a brief illness , died February 21, 1926 in Leiden .
With him Humanity has lost a great man in the broadest and deepest sense of the word.
We came to the final part of our humble work which, by successive steps, seeks to highlight the efforts of researchers to understand the laws that govern the gas at temperatures close to absolute zero.
We have found that French scientists, Dutch, English , Poles, Germans and others have contributed to the advancement of this science. And so it must be. It is true that physics is the prerogativeof a few men, but the cultural heritage that flows from it is part of humanity.
And perhaps therein lies the true strength of the sciences as real man's prerogative as such.

